Within six years, several patents from the pharmaceutical industry’s best-selling drugs are set to expire, leaving significant gaps in patent portfolios for pharmaceutical giants and creating opportunities for more competitive production in the space. Among the companies most impacted are Abbvie, Merck, Pfizer, and Novo Nordisk, with multi-billion-dollar revenue-generating drugs expiring by 2029.
Anticipating the impact made by their impending patent cliffs, pharmaceutical companies are aggressively looking for ways to generate new revenue through M&A activity, internal new drug development, protecting and profiting from new delivery methods for the expiring drugs, and more while still fending off the competition.
Competition in the industry will intensify with companies looking to maintain (or increase) their market share. What’s at stake are several billions of dollars of revenue opening to the broader pharmaceutical industry every few years.
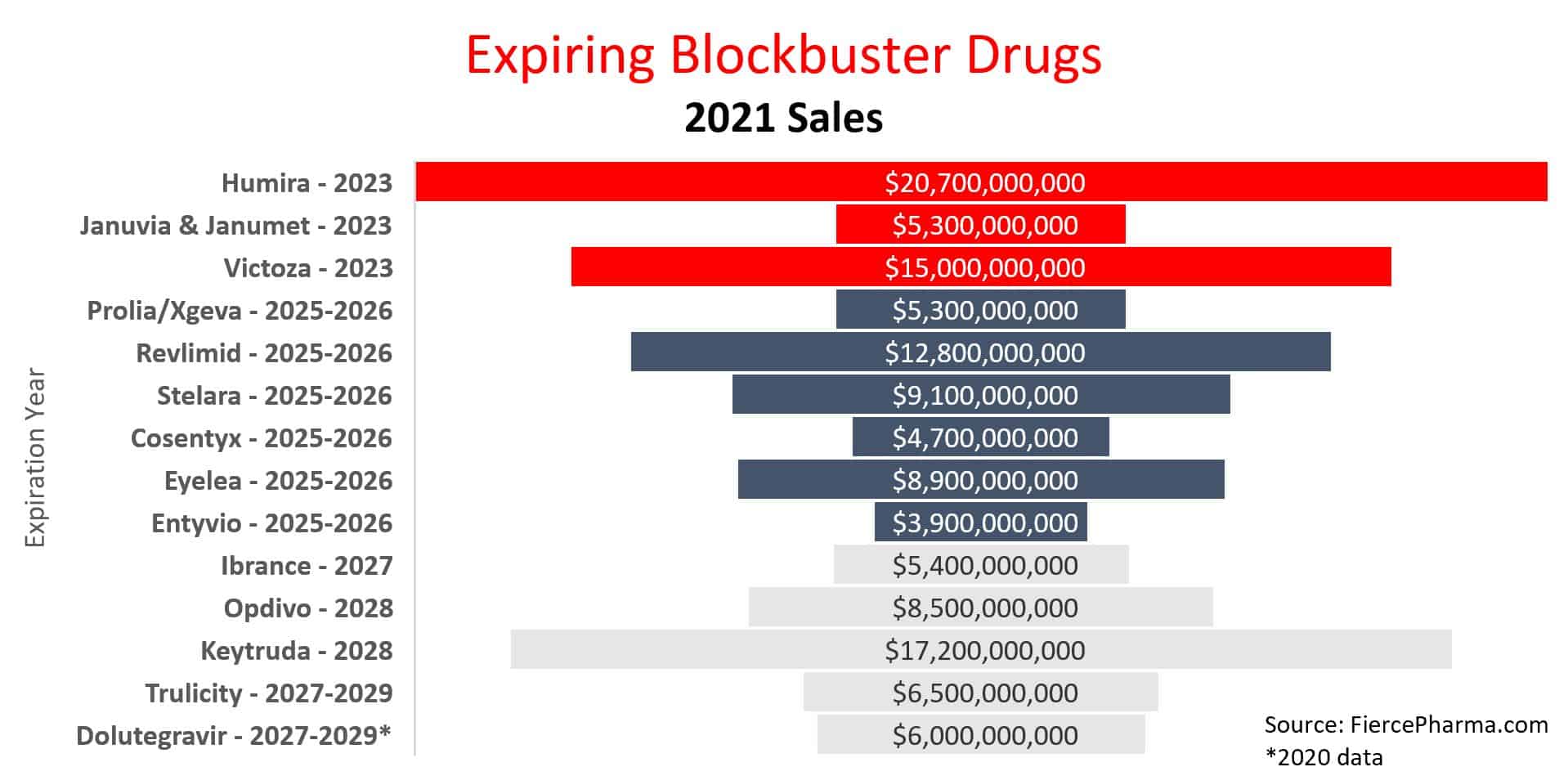
The value of the drugs listed above demands that the remaining exclusivity period is vigorously defended. WIT expects litigation to continue (and expand) for many of these drugs as the numbers are too enticing for competition to wait until expiration. A combination of Hatch-Waxman and antitrust litigation is becoming common, resulting in numerous ongoing battles to protect a drug in the market. The litigation numbers continue to show that revenue equals challenges. According to data reported on Lex Machina, one-third of all ANDA cases filed from 2020-2023 concern just six expiring blockbuster drugs.
With all this considered, it would be remiss to ignore the impact that ongoing ANDA conflicts and competition prompted by impending patent cliffs may have on the life sciences industry at large. So how can players in the space succeed?
With the high-stakes nature of ANDA litigation, ANDA lawyers and their clients should safeguard their strategies by engaging with a diverse team of experts.
A diverse team of experts can utilize their technical knowledge to help counsel navigate disputes concerning the underlying science of specific drug patents; questions regarding the safety, efficacy, and bioequivalence of the generic drug; and pharmaceutical industry best practices.
Retaining highly skilled technical experts, including chemists, chemical engineers, biologists, and pharmacologists, to assess patent portfolios can give counsel critical insight into their position in the market. In addition, these experts can provide guidance on claim construction, help navigate claim challenges, and identify monetization opportunities by highlighting the most valuable assets and the biggest vulnerabilities.
Technical experts can also work with former FDA and other regulatory experts to advise counsel on drug development and approval concerns relating to safety protocols and clinical trials to ensure drug developers are in compliance with standards and regulations.
On the other side of the aisle, brand pharmaceutical companies can engage with experts to stay competitive.
As industry players set their sights on M&A deals, a highly skilled expert can conduct due diligence and advise counsel on which patents present the clearest ROI. In the long run, engaging with experts can provide strategic advice on how to properly proceed with potential consolidations.
At WIT, our teams of pharmaceutical and life sciences experts are highly credentialled, many of whom are former pharmaceutical executives whose experience spans everything from product development to corporate transactions. Contact us to learn more about how our experts can support your patent portfolio strategy.




